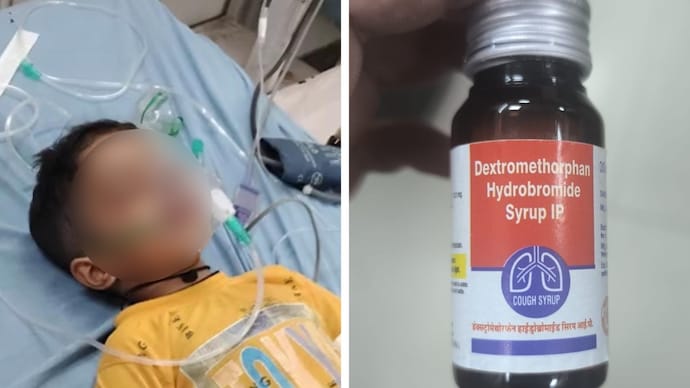
Cough syrup row: Two states, different actions after children’s deaths
Reports emerged in Madhya Pradesh and Rajasthan of tragic child deaths within days associated with use of certain paediatric cough syrups. The deaths triggered intense public concern and state-level regulatory responses. Rajasthan promptly suspended the state drug controller and withdrew multiple batches of the implicated syrup made by Kayson Pharma, while MP authorities have imposed bans locally, collected samples for testing, and faced scrutiny over regulatory lapses. The central health ministry says preliminary tests found no toxins associated with kidney injury in the syrup samples.
1. Child fatalities in MP and Rajasthan
In Madhya Pradesh’s Chhindwara district, several children developed kidney failure in quick succession after being treated for cough/fever with syrup. Officials suspect the syrup could be linked.
In Rajasthan, two children (ages two and five) died after being given a dextromethorphan-based syrup; at least ten others in the state fell ill.
2. Suspension and bans of batches
Rajasthan swiftly banned 22 batches of the syrup manufactured by Kayson Pharma, and halted distribution of 19 medicines from that company. The state also suspended its drug controller for alleged malfeasance.
Madhya Pradesh, meanwhile, banned use of two specific cough syrups locally and began collecting samples to test quality.
3. Central health ministry steps in
The Union Health Ministry / DGHS issued an advisory on cautious use of pediatric cough syrups. Also, it was reported that in preliminary analysis, no toxins known to cause kidney injury were found in tested samples of the suspected syrup.
4. Company under scrutiny
Kayson Pharma, the firm whose syrup is implicated (used under Rajasthan’s free medicine scheme), is now under close investigation. Authorities are probing all medicines made by it.
5. State disclaimers and counterclaims
Rajasthan’s health department clarified that the state’s free-medicine-distributed syrup may not be the cause of deaths in all cases. They assert that the specific batches causing harm may not include the ones supplied via the free scheme.
FAQs
Q1: Which states are involved in the cough syrup deaths, and what actions have they taken?
A1: The states are Madhya Pradesh and Rajasthan. Rajasthan suspended its state drug controller, ceased distribution of implicated batches from Kayson Pharma, and initiated a public probe. MP has banned certain syrups locally in affected districts, collected samples for lab testing, and is awaiting results before broader action.
Q2: Which company’s syrup is under suspicion, and what is being investigated?
A2: The company is Kayson Pharma (which supplied a dextromethorphan-based cough syrup). Authorities are investigating all medicines from the firm, especially those used in Rajasthan’s free medicine scheme.
Q3: What has the Union Health Ministry reported about toxicity in syrup samples?
A3: The Union Health Ministry has said that in preliminary analyses, the tested syrup samples did not contain toxins known to cause kidney injury.
Q4: What are the challenges to proving a medicine caused the deaths?
A4: Challenges include establishing causality (linking dosage and timing), ensuring sample integrity, detecting specific toxins, ruling out other causes of kidney failure, and navigating legal liability across manufacturer, distributor, regulator and clinicians.
Q5: What reforms should follow to prevent such tragedies?
A5: Key reforms include:
• Enhancing drug testing and laboratory infrastructure
• Mandating serialization / batch tracking
• Real-time recall mechanisms
• Transparent disclosures of lab findings
• Empowering state & central regulators with stricter oversight
• Clear prescribing guidelines and surveillance in rural health settings.