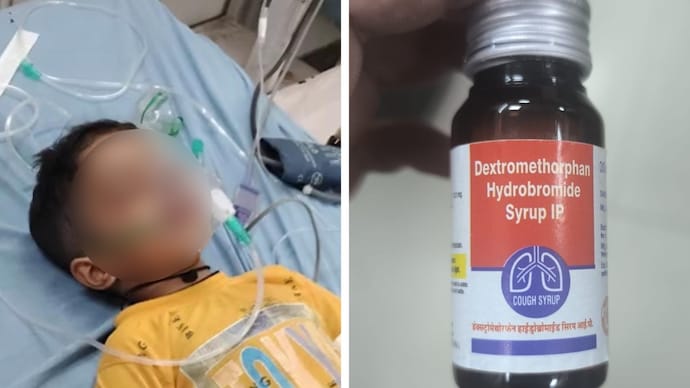
Manipur Bans Two Cough Syrup Brands After Toxic Chemical Found in Samples
The Manipur Drugs Control Administration has ordered an immediate ban and recall of two cough syrup brands — Relife and Resipfresh TR — after laboratory tests by the Madhya Pradesh Government Drug Testing Laboratory detected diethylene glycol, a highly toxic industrial solvent, in samples. Distributors, pharmacies, and retailers must withdraw affected batches and report compliance; health officials have been asked to watch for poisoning cases.
• The Manipur Drugs Control Administration issued a public alert and ordered the ban and recall of two cough syrup brands: Relife and Resipfresh TR.
• Tests by the Madhya Pradesh Government Drug Testing Laboratory reportedly detected diethylene glycol in samples of both syrups.
• The Drugs Control Department instructed distributors, pharmacies, and retailersacross Manipur to withdraw affected batches immediately and report compliance.
• Health officials have stepped up surveillance at medical stores and hospitals, and healthcare providers were warned to be vigilant for possible DEG poisoning cases among patients who may have consumed the syrups.
Frequently Asked Questions
Q1: Which cough syrup brands were banned in Manipur?
The Manipur Drugs Control Administration banned Relife and Resipfresh TR after lab tests detected diethylene glycol in samples. The ban includes an immediate recall and withdrawal of affected batches.
Q2: What chemical was found in the syrup samples and why is it dangerous?
Laboratory tests reportedly found diethylene glycol (DEG), a toxic industrial solvent that can cause acute poisoning, kidney failure, and death if ingested. DEG is not a medicine ingredient and should never be present in oral formulations.
Q3: What should I do if I’ve used these syrups recently?
Stop using the brands immediately. Check the batch number and, if you suspect your bottle is part of the recall, return it to the pharmacy or follow the Drugs Control Department’s instructions. If you or someone who consumed it feels unwell (vomiting, abdominal pain, reduced urine output), seek emergency medical care and inform clinicians about possible syrup exposure.
Q4: Are regulators testing other medicines now?
When a contaminant like DEG is detected, regulators typically expand testing to related products, suppliers, and batches to determine the contamination scope. Manipur officials have intensified surveillance across medical stores and hospitals as part of this process.
Q5: How can consumers protect themselves from contaminated medicines in general?
Buy medicines from licensed pharmacies, check packaging and batch numbers, keep receipts, avoid repackaged items, and follow recall notices from authorities. Report any adverse reactions immediately to healthcare providers and regulators.